
2021 Gmc Sierra 10-speed Transmission Problems, 2020 Silverado 1500 10 speed transmission issues, 7.87 MB, 05:44, 38,791, crkdtoes, 2021-01-25T22:55:06.000000Z, 19, New 2021 Cayenne Red Tintcoat GMC Sierra 1500 Crew Cab Short Box 4, www.taylorsautomaxbuickgmc.com, 960 x 540, jpeg, at4 denali buick yakima slt cayenne tintcoat peterson trim ritchey chateauguay daytona jerseyville elevation sle vin, 12, 2021-gmc-sierra-10-speed-transmission-problems, KAMPION
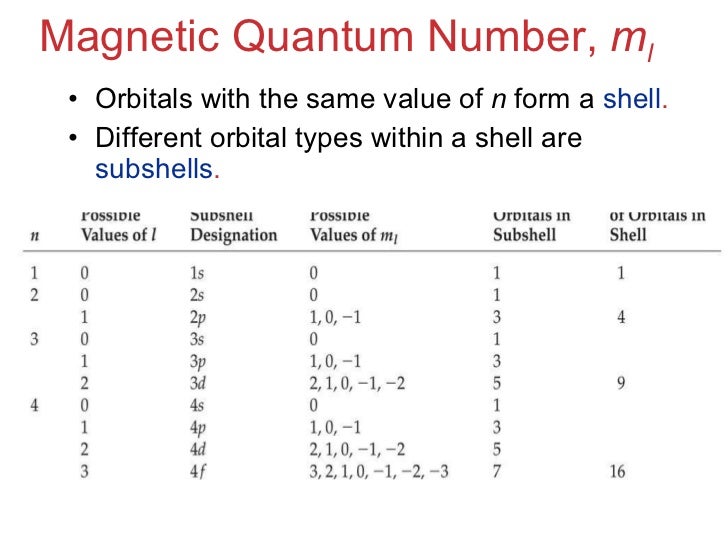
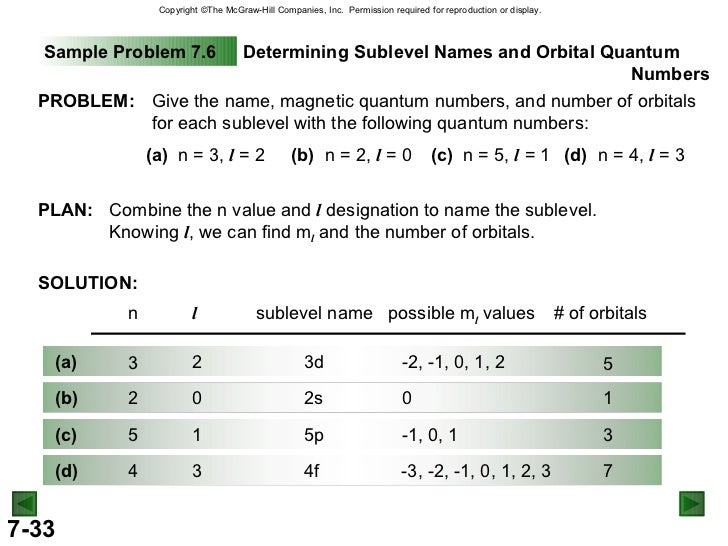
0 , 1, 2, 3, 4. How many liters of o2 gas, measured at 781 mmhg and 24 ∘c , are required to completely react with 2. 7 mol of al ? And so with m l being 3, well there's constraints on what m l can be and if l was 3, m l can be starting at negative whatever l is so negative 3 and then increasing by 1's so negative 2, negative 1, 0 and 1, 2 up to a maximum of l which is 3 so hypothetically, if l was 3, that would allow an m l. So we give them the values for the quantum numbers n and l of a sub shell off five and three, respectively, on were asked to work out the possible values off the quantum numbers.
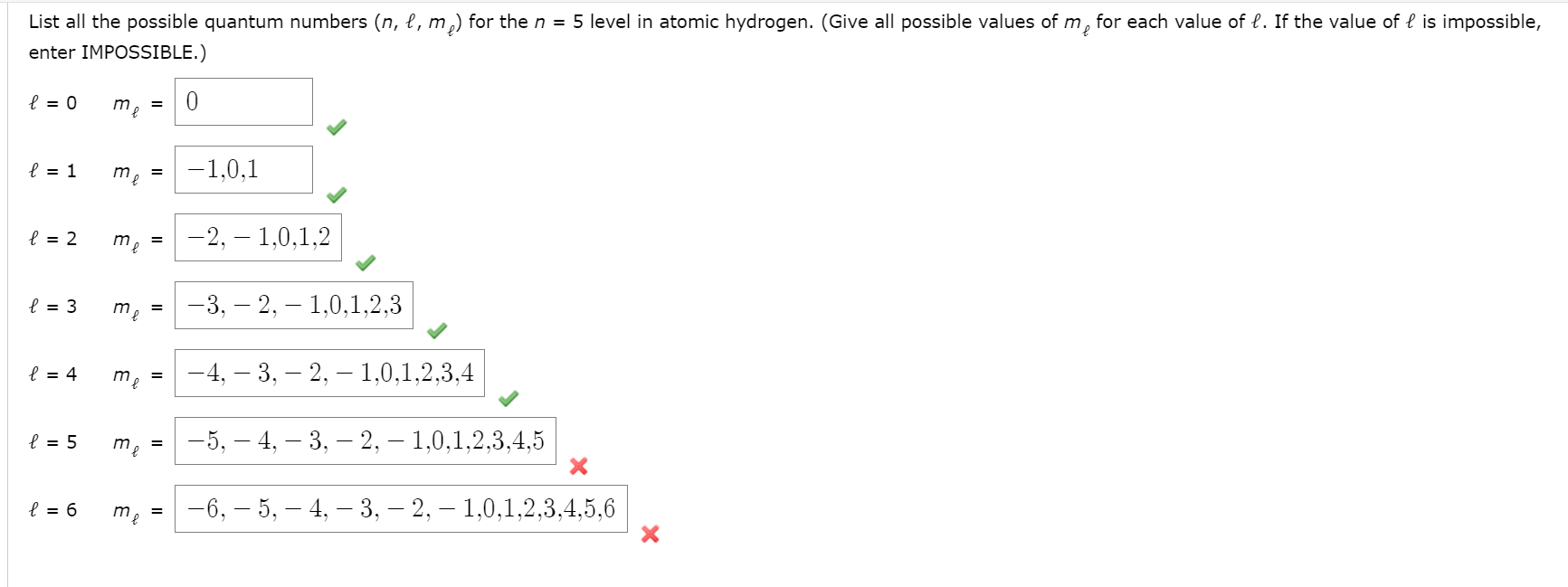
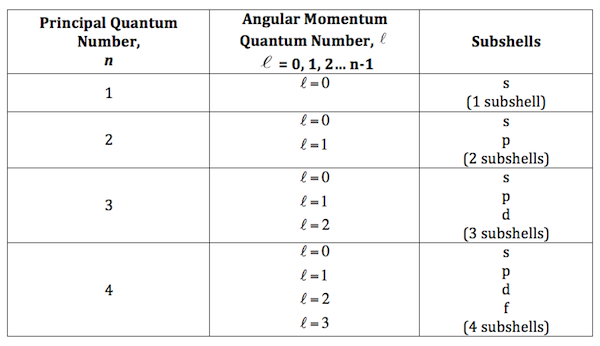
💬 👋 we’re always here. Join our discord to connect with other students 24/7, any time, night or day. join here! The sum of all possible values of l and m for n is is equal to is. There are four different types of quantum numbers used to decsribe an electron present in an orbital. ; They are principal quantum number,azimuthual quantum number,magnetic quantum number and spin quantum number. ; Principal quantum number gives us information about the size and. (a) the orbital angular momentum quantum number (l) determines the shape of an orbital. for any given orbital, this quantum number can have positive integral values from zero up to one less than the value of n for the orbitals. For any given orbital, l can have values ranging from 0, 1, 2…. ,. So for n = 5, the possible values of l are
[Explained] What Are The Possible Values Of The Angular Momentum
![What Are The Possible Values Of L If N = 5? [Explained] What Are The Possible Values Of The Angular Momentum](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEh0iF6yb3UhthPfoMwtcSL4aX7zmDEqFpQW6F49DpwUBAe52tV5ZrHdQv0Q-bwuaMaldqB1yFOqgJaGOCv95sPRaGhL5YWXqUN2kppFqRd8gs9uxpnpTn6aK14TI4JOG8HhgNlPiKPJ-BPC/s810/Permissible+Possible+combinations+of+principal+azimuthal+quantum+numbers.jpg)
Solved: List All The Possible Quantum Numbers (n, L, M,) F... | Chegg.com
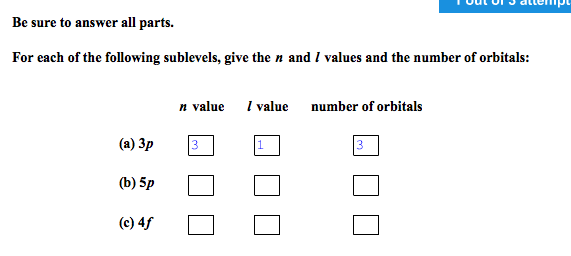
Solved: Be Sure To Answer All Parts. For Each Of The Follo... | Chegg.com
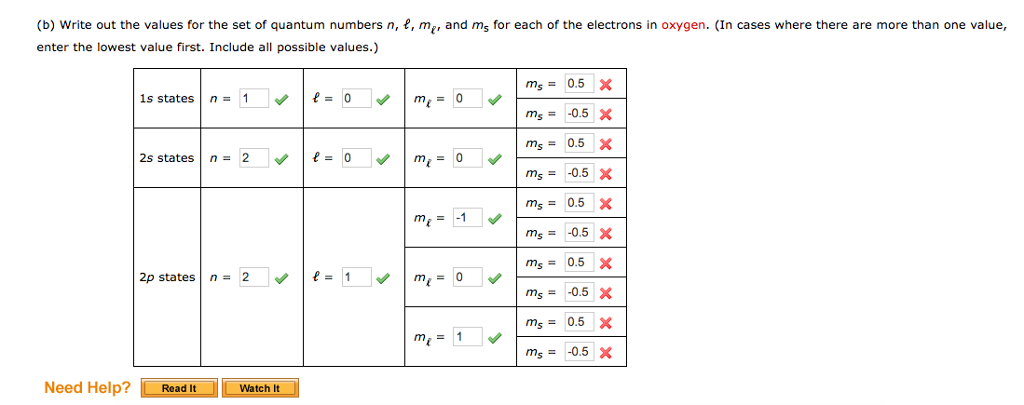
Solved: Write Out The Values For The Set Of Quantum Number... | Chegg.com
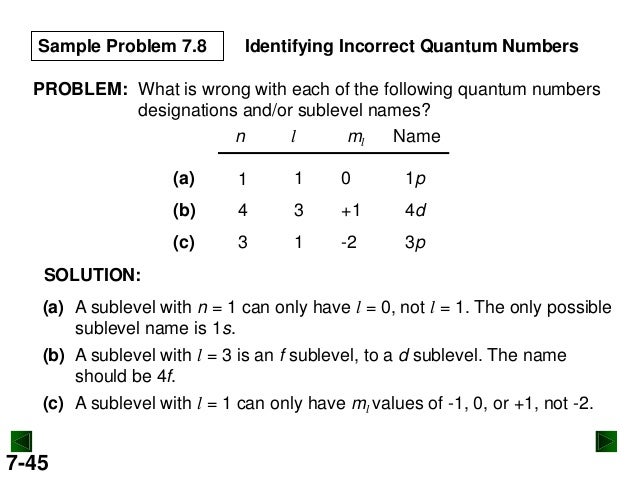
New chm 151_unit_4_power_points
Orbitals
Chapter 6 Lecture- Electrons in Atoms
Electronic structure
Interactive Student Tutorial
Quantum Numbers - Azimuthal Quantum Number | Types of Quantum Numbers
EmoticonEmoticon