2021 Gmc Sierra 10-speed Transmission Problems, 2020 Silverado 1500 10 speed transmission issues, 7.87 MB, 05:44, 38,791, crkdtoes, 2021-01-25T22:55:06.000000Z, 19, New 2021 Cayenne Red Tintcoat GMC Sierra 1500 Crew Cab Short Box 4, www.taylorsautomaxbuickgmc.com, 960 x 540, jpeg, at4 denali buick yakima slt cayenne tintcoat peterson trim ritchey chateauguay daytona jerseyville elevation sle vin, 12, 2021-gmc-sierra-10-speed-transmission-problems, KAMPION
Each shell can contain only a fixed number of electrons: The first shell can hold up to two electrons, the second shell can hold up to eight (2 + 6) electrons, the third shell can hold up to 18 (2 + 6 + 10) and so on. The general formula is that the. the outer shell has 5 electrons, it requires 3 more electrons to complete the outer energy shell.
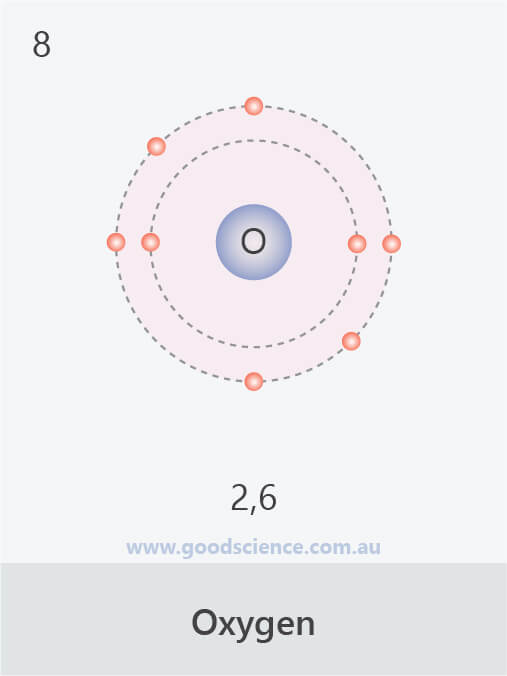
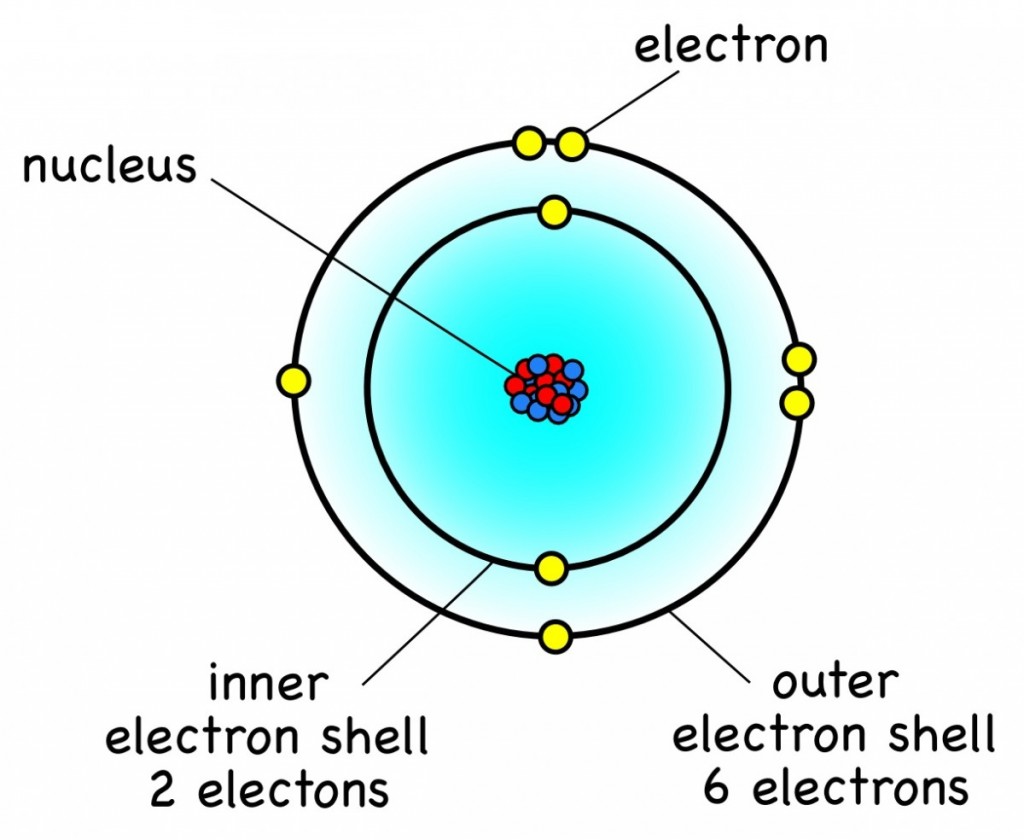
Nitrogen has atomic number = 7. The first shell holds 2 electrons. Nitrogen has atomic number = 7. Nitrogen, atomic #7, has 5. Carbon (c), en masse 14 component, has 4 electrons in its outershell carbon generally shares electrons to attain a full valence shell, generating bonds with many various various other atoms. As a result, the columns of the table of elements mirror the number of electrons located in each component’s valence shell, which therefore creates how. That is, the number of electrons in oxygen is 8. Therefore, an oxygen atom will have two electrons in the first shell and six in the 2nd shell. Therefore, the order of the number of electrons in each shell of the oxygen(o) atom is 2, 6.
Valency is the number of bonds an atom can make with others
Electron Configuration (Elements 1-20) | Good Science
Revise Electron Arrangements – S3 Chemistry Consolidation
This four panel figure shows four different atoms with the electrons in
chapter 02
Electronic Configuration | Distribution of Electrons | Chemistry

Chemistry online journal(≧ ≦)*(^o^)*: Atomic structure

savvy-chemist: GCSE OCR Gateway Chemistry C2.2 d-i Bonding and the

The top panel in this figure shows two hydrogen atoms sharing two
Atoms, Isotopes, Ions, and Molecules: The Building Blocks | OpenStax

EmoticonEmoticon