
2021 Gmc Sierra 10-speed Transmission Problems, 2020 Silverado 1500 10 speed transmission issues, 7.87 MB, 05:44, 38,791, crkdtoes, 2021-01-25T22:55:06.000000Z, 19, New 2021 Cayenne Red Tintcoat GMC Sierra 1500 Crew Cab Short Box 4, www.taylorsautomaxbuickgmc.com, 960 x 540, jpeg, at4 denali buick yakima slt cayenne tintcoat peterson trim ritchey chateauguay daytona jerseyville elevation sle vin, 12, 2021-gmc-sierra-10-speed-transmission-problems, KAMPION
All particles are attracted to each other, but some combinations of particles are attracted more strongly than others. In an ionic co. One reason ionic compounds do not dissolve well in nonpolar solvents is that o there are no forces of attraction between ions and nonpolar molecules. Not all cations and anions have the same magnitude of charge and therefore do not.
(pe1) one reason ionic compounds do not dissolve well in nonpolar solvents is that. B) there are no forces of attraction between ions and nonpolar molecules. C) not all cations and anions have the same magnitude of charge and therefore do not form neutral ion pairs. Click here 👆 to get an answer to your question ️ one reason ionic compounds do not dissolve well in nonpolar solvents is that besapoo3phis besapoo3phis 02/19/2017 C) not all cations and anions have the same magnitude of charge and therefore do not form. It isn't strictly true, but generally ionic compounds are not highly soluble in organic solvents because ionic compounds need a highly polar solvent to dissolve well (such as water) and in general. One reason ionic compounds do not dissolve well in nonpolar solvents is that a. Not all cations and anions have the same magnitude of charge and therefore do not form neutral ion pairs. They can dissolve only in polar compounds.
Answered: One reason ionic compounds do not… | bartleby

Answered: Question 6 AHvap is the slope of a plot… | bartleby

What is the meaning of the “like dissolve like” rule in chemistry? - Quora
(Solved) One reason ionic compounds do not dissolve well in nonpolar
Ionic Liquids
Chapter 3: Liaisons and molecular orbitals | Borzuya university
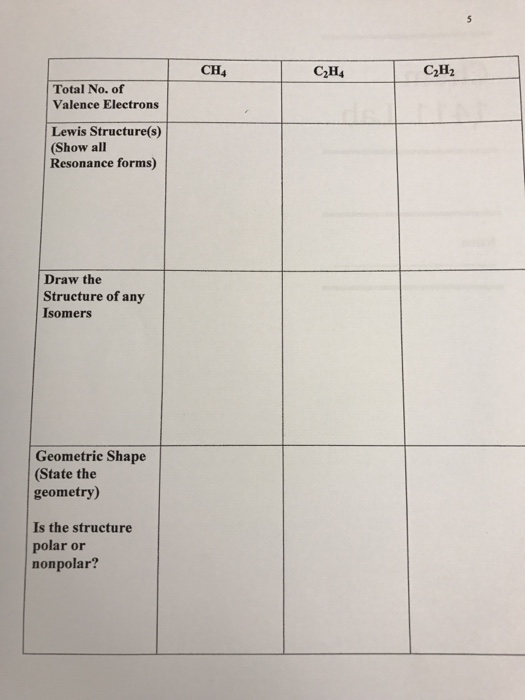
Ch4 Polar Or Nonpolar / Free Online Help: how do you figure about
EmoticonEmoticon