
2021 Gmc Sierra 10-speed Transmission Problems, 2020 Silverado 1500 10 speed transmission issues, 7.87 MB, 05:44, 38,791, crkdtoes, 2021-01-25T22:55:06.000000Z, 19, New 2021 Cayenne Red Tintcoat GMC Sierra 1500 Crew Cab Short Box 4, www.taylorsautomaxbuickgmc.com, 960 x 540, jpeg, at4 denali buick yakima slt cayenne tintcoat peterson trim ritchey chateauguay daytona jerseyville elevation sle vin, 12, 2021-gmc-sierra-10-speed-transmission-problems, KAMPION
Mg has paramagnetic property, in spite of not having single electron in its configuration and gold shows diamagnetic property in spite of having single electron in its s orbital. To determine whether the elements are paramagnetic or diamagnetic, write out the electron configuration for each element. 1s 2 subshell is filled. 1s 2 2s 2 subshell is filled.
1s 2 2s 2 2p 3 subshell is not filled. Diatomic gases are also almost exclusively diamagnetic, and not paramagnetic. Magnesium like most metals is diamagnetic. Some substances exhibiting diamagnetic behaviour can switch to the paramagnetic state when the temperature is increased. Solution for is magnesium paramagneic or diamagnetic and why? Is vanadium paramagneic or diamagnetic and why? First week only $4. 99! We've got the study and writing resources you need for your assignments. Magnesium is described as paramagnetic meaning it is weakly attracted by a magnet and cannot be permanently magnetised.
Solved: Ral Diamagnetic Species, Which Are Not Attracted T... | Chegg.com

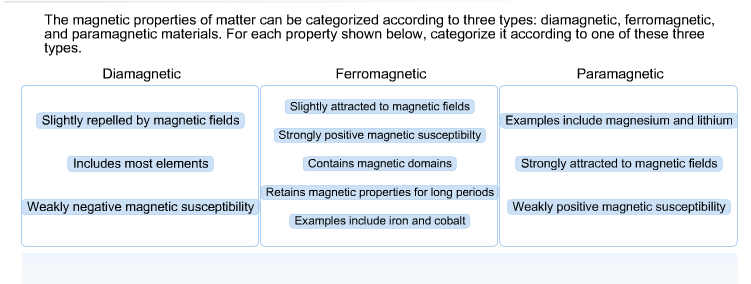
Solved: The Magnetic Properties Of Matter Can Be Categoriz... | Chegg.com
Is magnesium diamagnetic or paramagnetic? Why? - Better This World

[6] (a) Write an orbital diagram for the ground state of Magnesium (Mg
![Is Magnesium Diamagnetic Or Paramagnetic? Why? [6] (a) Write an orbital diagram for the ground state of Magnesium (Mg](https://img.homeworklib.com/images/b4358686-1dc6-4c4c-9ed3-6dd71a4b0aa1.png?x-oss-process=image/resize,w_560)
Spectroscopic and Magnetic Properties of Coordination Compounds | Chemistry

PPT - Electron Configurations Chemical Periodicity (Ch 8) PowerPoint
| Molecular orbitals and magnetic response of triplet and singlet

Paramagnetic character is observed in(2) 02(1) O(3) Naliq. NH3(4) All

[6] (a) Write an orbital diagram for the ground state of Magnesium (Mg
![Is Magnesium Diamagnetic Or Paramagnetic? Why? [6] (a) Write an orbital diagram for the ground state of Magnesium (Mg](https://img.homeworklib.com/images/d5c7bdfe-914e-4998-90e7-d7bc5bf52ea3.png?x-oss-process=image/resize,w_560)
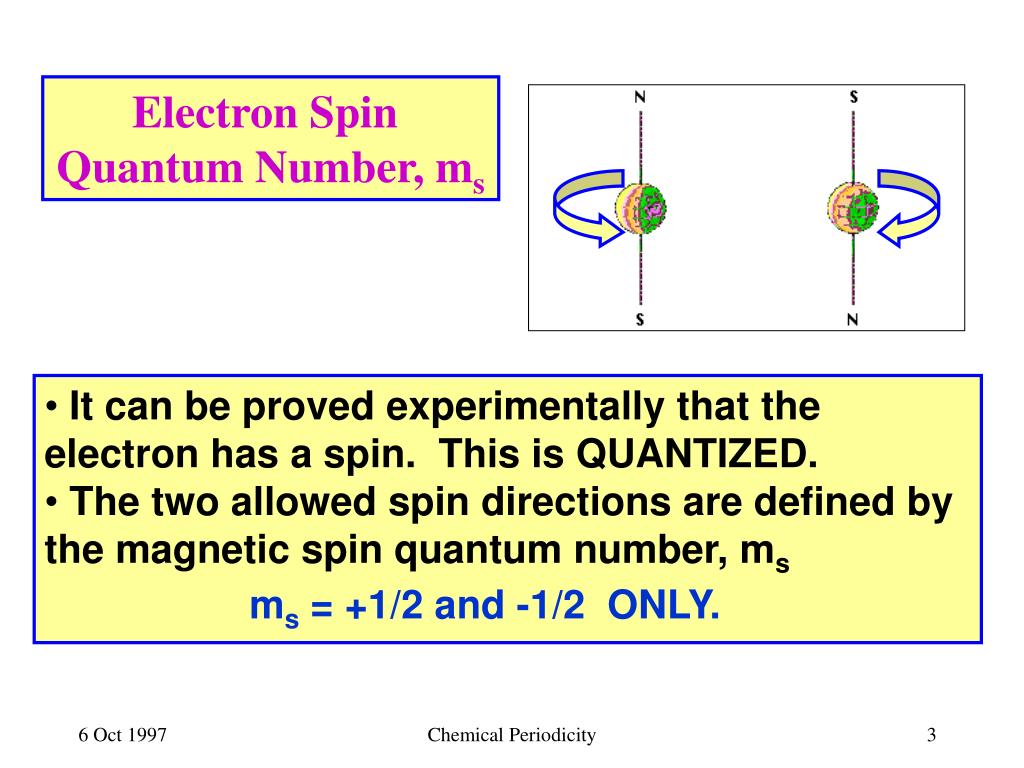
PPT - Sections 7.1 – 7.3 Electron Spin, Orbital Energies and Electron



EmoticonEmoticon